Why Does Vapor Pressure Increase With Temperature . the effect of temperature on the saturated vapor pressure of water. The line on the graph shows the boiling. The natural logarithm (ln) changes the nonlinear. The pressure scale (the vertical one) is. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. chemistry phases of matter vapor pressure and boiling. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c.
from saylordotorg.github.io
the effect of temperature on the saturated vapor pressure of water. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The natural logarithm (ln) changes the nonlinear. The line on the graph shows the boiling. The pressure scale (the vertical one) is. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. chemistry phases of matter vapor pressure and boiling. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water.
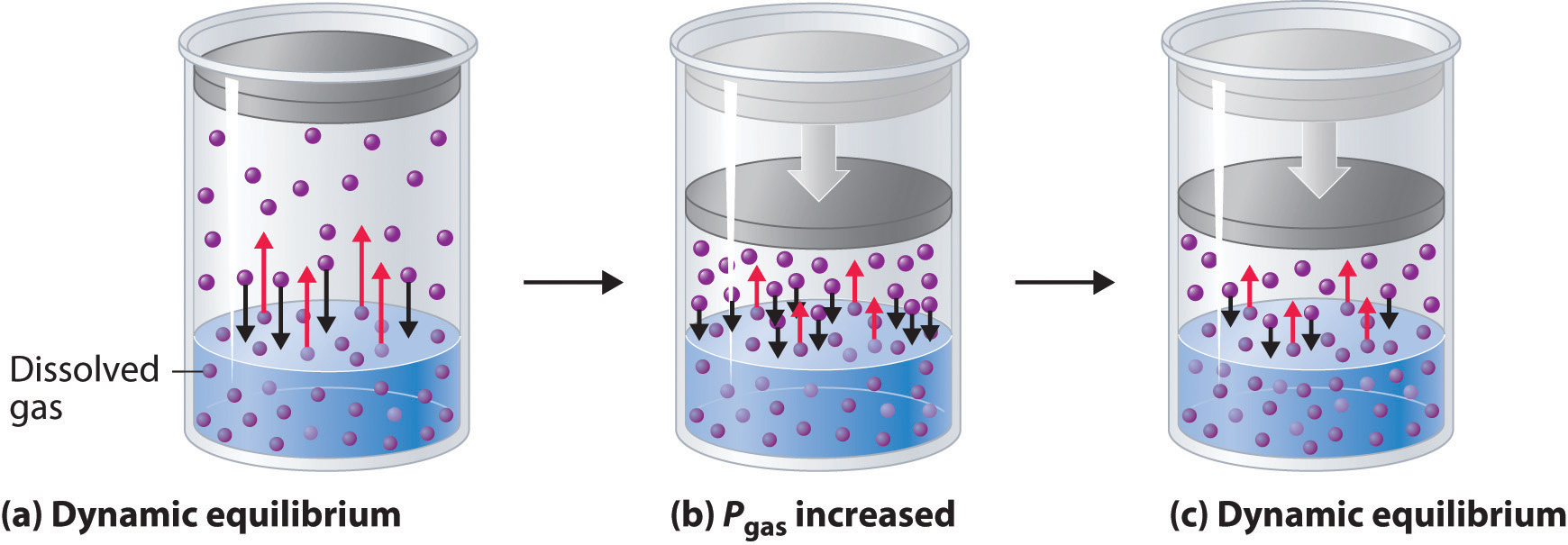
Effects of Temperature and Pressure on Solubility
Why Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The pressure scale (the vertical one) is. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the effect of temperature on the saturated vapor pressure of water. The natural logarithm (ln) changes the nonlinear. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. chemistry phases of matter vapor pressure and boiling. The line on the graph shows the boiling. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c.
From userenginepilches.z14.web.core.windows.net
Phase Diagrams Understanding The Basics Why Does Vapor Pressure Increase With Temperature The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. chemistry phases of matter vapor pressure and boiling. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The natural logarithm (ln) changes the nonlinear. generally a substance's vapor pressure increases as temperature. Why Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 10Solutions PowerPoint Presentation, free download ID Why Does Vapor Pressure Increase With Temperature the effect of temperature on the saturated vapor pressure of water. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The line on the graph shows the boiling. chemistry phases of matter vapor pressure and boiling. The natural logarithm (ln) changes the nonlinear. the vapor pressure of a liquid. Why Does Vapor Pressure Increase With Temperature.
From www.chegg.com
Solved Question 5 Why does vapor pressure increase with an Why Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the effect of temperature on the saturated vapor pressure of water. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The graph shows how the saturated. Why Does Vapor Pressure Increase With Temperature.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Why Does Vapor Pressure Increase With Temperature the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The pressure scale (the vertical one) is. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the effect of temperature on the saturated vapor pressure of water. generally a substance's vapor pressure. Why Does Vapor Pressure Increase With Temperature.
From partmcveighinjudicial.z21.web.core.windows.net
Phase Diagram Generator Why Does Vapor Pressure Increase With Temperature The natural logarithm (ln) changes the nonlinear. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. chemistry phases of matter vapor pressure and boiling. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. generally a substance's vapor pressure increases as temperature. Why Does Vapor Pressure Increase With Temperature.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Why Does Vapor Pressure Increase With Temperature The pressure scale (the vertical one) is. The line on the graph shows the boiling. The natural logarithm (ln) changes the nonlinear. the effect of temperature on the saturated vapor pressure of water. chemistry phases of matter vapor pressure and boiling. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c.. Why Does Vapor Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Why Does Vapor Pressure Increase With Temperature The natural logarithm (ln) changes the nonlinear. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The line on the graph shows the boiling. chemistry phases of matter vapor pressure and boiling. The pressure. Why Does Vapor Pressure Increase With Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor Why Does Vapor Pressure Increase With Temperature chemistry phases of matter vapor pressure and boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the effect of temperature on the saturated vapor pressure of water. the vapor pressure of. Why Does Vapor Pressure Increase With Temperature.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Why Does Vapor Pressure Increase With Temperature the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the effect of temperature on the saturated vapor pressure of water. The pressure scale (the vertical one) is. The natural logarithm (ln) changes the. Why Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Why Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. the effect of temperature on the saturated vapor pressure of water. generally a substance's. Why Does Vapor Pressure Increase With Temperature.
From www.askiitians.com
Raoults Law Study Material for IIT JEE askIITians Why Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. The pressure scale (the vertical one) is. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The natural logarithm (ln) changes the nonlinear. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the graph. Why Does Vapor Pressure Increase With Temperature.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Why Does Vapor Pressure Increase With Temperature The pressure scale (the vertical one) is. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The natural logarithm (ln) changes the nonlinear. chemistry phases of. Why Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Why Does Vapor Pressure Increase With Temperature The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. chemistry phases of matter vapor pressure and boiling. The pressure scale (the vertical one) is. The line on the graph shows the boiling. Web. Why Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Why Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. the effect of temperature on the saturated vapor pressure of water. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The natural logarithm (ln) changes the nonlinear. The pressure scale (the vertical one) is. the vapor pressure of a liquid varies with its. Why Does Vapor Pressure Increase With Temperature.
From www.numerade.com
From the plot of vapor pressures vs temperature above, estimate the Why Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The line on the graph shows the boiling. The pressure scale (the vertical one) is. The graph shows how the saturated vapor pressure (svp) of water. Why Does Vapor Pressure Increase With Temperature.
From www.nuclear-power.com
Temperatureentropy Diagrams Ts Diagrams Why Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. chemistry phases of matter vapor pressure and boiling. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The natural logarithm (ln) changes the nonlinear. the graph shows how the saturated vapor pressure (svp) of. Why Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Why Does Vapor Pressure Increase With Temperature The pressure scale (the vertical one) is. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. chemistry phases of matter vapor pressure and boiling. The line on the graph shows the boiling.. Why Does Vapor Pressure Increase With Temperature.
From www.youtube.com
Vapor Pressure and Boiling YouTube Why Does Vapor Pressure Increase With Temperature the effect of temperature on the saturated vapor pressure of water. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The line on the graph shows the boiling. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. the vapor pressure of a liquid. Why Does Vapor Pressure Increase With Temperature.